
0 introduction
Quartz glass is made of a single silica (SiO2It has the advantages of high melting point, small expansion coefficient and high ultraviolet transmittance, and is a key basic material for electric light sources, photovoltaic industry and semiconductors. In recent years, the rapid development of the photovoltaic industry and the semiconductor industry has led to a rapid increase in the output of quartz glass in China, but because the quartz glass industry has not yet established an independent waste recycling and reuse system, a large number of new quartz glass waste has entered the raw material link of the broken glass of building float glass, which has caused new problems in the production of building float glass industry. In the first half of 2024, the frequency of such problems in the production of building float glass in China will increase significantly, so it is necessary to carry out relevant research on the solid phase defects caused by quartz glass, so as to provide a clear reference for glass companies to judge and solve such problems.
1 Characteristics of quartz glass
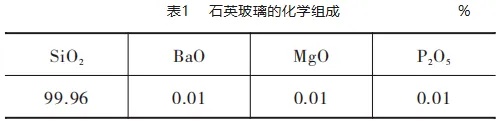
Quartz glass has a melting temperature of 1740 °C and a softening temperature of 1600 °C. The hot spot temperature in the float glass melting furnace is generally set at about 1600 °C, and it is difficult for the quartz glass mixed in the raw material to be completely melted after entering the float glass melting furnace. Quartz glass will be left in the high temperature environment of the glass furnace for a certain period of time, and some or all of it will turn into white crystals. The density of quartz glass is 2.21 g/cm3, which is smaller than that of ordinary float soda-lime silicate glass of 2.51 g/cm3Therefore, the quartz glass mixed in the raw material broken glass will usually turn into a white siliceous non-melt, float on the liquid glass surface, and drift with the production flow to the neck water bag. Table 1 shows the chemical composition of quartz glass sorted out in raw material cullet by a float glass company, and the test equipment is Panalytical's X-ray fluorescence spectrometer with model Axios-MAX.
1) Morphological characteristics of solid phase defects caused by quartz glass
Fig. 1 shows the solid phase defect caused by quartz glass picked out at the stuck neck water bag of the float glass melting furnace.


Fig.1. Solid phase defects caused by quartz glass
Figure 1(a) retains more information about the quartz glass, including the original shape of the quartz glass, the layered structure of the upper and lower surfaces and the middle phase of the glass phase. Fig. 1(b) shows the solid phase defect formed by the stagnation of quartz glass for a long time at high temperature, and its whole body completely transforms into white quartz crystal, and forms a more obvious plate shape due to the long-term floating on the molten glass.
(2) Component analysis of solid phase defects caused by quartz glass
For the solid phase defect in Figure 1(a), the sample was observed using an electron probe model number JEOL JXA-8230, and Figure 2 shows the electron image of the electron probe backscattered by the sample, where Spectra 1 and 2 are the glass regions, and Spectra 3, 4, 5, and 6 are the white areas of the solid phase defects. The yield of backscattered electrons in the electron microscope is correlated with the atomic number, and the deviation of the composition of each region can be reflected by the different brightness of each region, and the energy spectrum of each selected microregion of the solid phase defect is further quantitatively analyzed, and the specific data are shown in Table 2.


It can be seen from Table 2 that the overall SiO2 content of the solid phase defects is more than 99.00%, and the oxide composition of this defect is significantly different from that of siliceous sand and siliceous refractories. The composition analysis of the solid phase defects in Figure 1(b) with obvious diffusion and dissolution was performed using an X-ray fluorescence spectrometer with model Axios-MAX, and the test results are shown in Table 3.
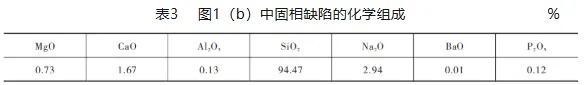
A comparison of Table 2 and Table 3 shows that the SiO2 content in Figure 1(b) is reduced to 94.47% Na2 compared to the solid defect composition in Figure 1(a). The O content is increased to 2.94% and the CaO content is increased to 1.67%, due to the higher degree of dissolution and diffusion of the quartz glass in the glass melt in Figure 1(b).
(3) Crystalline phase analysis of solid phase defects caused by quartz glass
The phase analysis of the above two solid phase defects was carried out using an X-ray diffractometer with RIgaku-Ultima IV., and the diffraction patterns of the two defects were basically the same, as shown in Figure 3. The diffraction pattern of the standard PDF card was found to be consistent with the characteristic peak positions of the cristobalite (SiO2) crystal. Compared with the solid phase defects caused by unfused silica sand and siliceous refractories, there are few phosphoquartz occurrences in the defects caused by quartz glass impurities, and there are no α-quartz and β- The presence of low-temperature crystalline phases such as quartz.
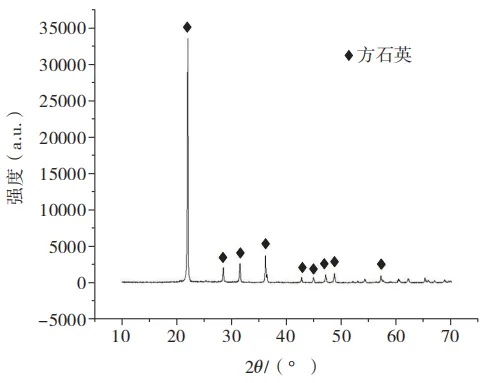
Fig.3. X-ray diffraction pattern of solid phase defects induced by quartz glass
2 Results & Analysis
After entering the float glass melting furnace, the quartz glass impurities in the cullet usually float on the upper layer of the molten glass and gradually crystallize, transforming from a transparent glass phase to a white opaque cristobalite crystal. When the melting temperature is low or the stagnant time in the furnace is short, the middle layer of quartz glass may still have a part of the glass phase, and the SiO2 content in its overall oxide composition exceeds 99.00%; When the melting temperature is high or the stagnation time in the furnace is long, the quartz glass will completely transform into a white opaque cristobalite crystal. In addition, due to the diffusion of alkali metal and alkaline earth metal ions in the float glass liquid, the overall oxide composition of the SiO2 content will be reduced to about 94.50%, and the Na2Ocontent will be increased to about 3.00%. Compared with siliceous stones formed by silica sand and siliceous refractories, the solid phase defects formed by quartz glass impurities usually have a higher SiO2 content and are mainly cristobalite crystalline phases, with less phosphoquartz and no α-quartzβ - Quartz
3 epilogue
The quartz glass mixed in the cullet will lead to a significant decrease in the yield after entering the glass melting furnace. In the specific production process, it is necessary to grasp the morphology, composition and crystalline phase characteristics of solid phase defects caused by impurities in quartz glass. When a similar problem occurs, the cause can be quickly determined based on the characteristics of the solid phase defect and the change of the material layer on the liquid glass surface in the furnace. In the daily production management, it is necessary to further strengthen the quality control of the purchased broken glass, in addition to the conventional sorting items such as dust, metal and colored glass, increase the sorting requirements for special-shaped ultra-white materials in the broken glass, and reduce the probability of quartz glass entering the furnace as much as possible, so as to effectively avoid the solid phase defects caused by quartz glass and improve product quality.
Name: Litong Glass
Mobile:+86 16632961602
Tel:+86 16632961602
Email:vip@litongglass.com
Add:Shahe city,Hebei,China